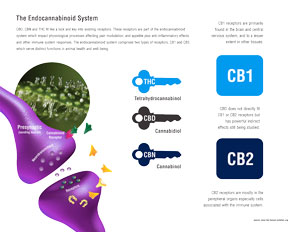
The endocannabinoid system (eCB) is an essential regulator of bodily function in its many facets. There is hardly any physiological process that is not affected by it to some degree. It is surprising then to realize that the eCB was totally unknown prior to one generation ago. The name derives from the fact that the bodies of all higher animals harbor natural chemicals within that resemble in many respects the activity of tetrahydrocannabinol (THC), the phyto- (plant) cannabinoid that is the main psychoactive component of Cannabis sativa, sometimes derisively labeled as marijuana. Despite the prominence and importance of the eCB as an essential regulatory mechanism in the body’s biochemistry and physiology, the basic machinery of everyday life, knowledge of it remains quite limited among American physicians due to a dearth of appropriate education in medical schools. This is a knowledge deficit that must be filled in order to benefit the public health as a whole.
The basic functions of the eCB were summarized in 1998 by Professor Di Marzo as, “relax, eat, sleep, forget and protect.” There are two primary endocannabinoids, arachidonylethanolamine (AEA), nicknamed anandamide from the Sanskrit word for “bliss,” and 2-arachidonylglycerol (2-AG). CB1 is best known as the neuromodulatory receptor in the brain where THC exerts its effects on short-term memory, pain, emotion, hunger, etc. Receptors may be thought of as locks, to which a corresponding chemical (natural or synthetic) will conform like a key, if it has the proper structure to conform to it. cB1 is actually the most abundant Gprotein attests to its importance in cerebral function in health and disease. Both endocannabinoids bind to cannabinoid receptors in a similar manner to THC in the brain, but are actually produced on demand in post-synaptic neurons(nerve cells) and travel in a retrograde fashion (backwards) to inhibit the release of various neurotransmitters (chemical messengers). As one example, neuropathic (nerve-based) pain is an all too common condition associated with multiple sclerosis, diabetes and HIV/AIDS, and which is notoriously difficult to treat with conventional pharmaceuticals. Glutamate is one of the primary stimulatory neurotransmitters, but when present at excessive concentrations, it perpetuates neuropathic pain and may even provoke cell death after head injury or stroke. The endocannabinoids are naturally secreted after such insults and act to inhibit glutamate release, thereby alleviating neuropathic pain and reducing cell death. THC, and cannabidiol (CBD), a non-psychoactive component of some cannabis strains, have similar neuroprotective benefits.
AEA and 2-AG are merely the star players in a larger group of endocannabinoids. Some of the others are seemingly inactive molecules when tested on their own. When combined with AEA and 2-AG, however, many experiments have demonstrated that these entourage compounds produce prominent enhancement of the overall effect on pain, inflammation or other function. This synergy (boosting) of effect due to an ensemble of ingredients has been termed the “entourage effect,” and is paralleled by similar attributes in the cannabis plant, whose minor components modulate (modify or influence) the effects of THC.
Beyond the brain, CB1 receptors are abundant in the spinal cord and peripheral nervous system, where they have a key role in regulation of pain, itch and muscle tone. The ECS also influences the gastrointestinal tract, where CB1 modulates two important aspects of digestion: propulsion and secretion. The endocannabinoid system also regulates endocrine function and fertility, as well as factors in cellular function, whether developmentally or in the uncontrolled growth and spread of cancer (see below).
CB1, however, is not the only cannabinoid receptor. Less studied, but extremely important is CB2, a non-psychoactive receptor that is mostly found in the periphery (outside the brain) and which is a key immunomodulatory mediator with additional activity on pain and inflammation. It, too, is expressed in the brain under conditions of insult, whether it be traumatic injury or degenerative diseases. Many disorders characterized by fibrosis (development of scar tissue), such as liver cirrhosis, and certain heart and kidney disorders may be targets for drugs that affect CB2.
A third receptor, TRPV1 (transient receptor potential vanilloid-one) is also considered part of the ECS, and is best known as the site of action of capsaicin, the active ingredient of chile peppers, but is also a target of anandamide and cannabidiol, but not THC. TRPV1 mediates pain signals through a mechanism distinct from that of the endogenous cannabinoids and opioids, but the receptor is subject to desensitization: this means that if continuously stimulated, the pathway will eventually slow down or even stop. This raises therapeutic possibilities for agents to effectively treat certain kinds of neuropathic pain.
The third component of the ECS along with the endocannabinoids and their receptors are the biosynthetic and degradative enzymes that respectively produce or breakdown AEA and 2- AG. These have also become targets for new drug development, and interestingly cannabidiol, among its many activities is capable of inhibiting AEA breakdown by the enzyme fatty acid amidohydrolase (FAAH), thus strengthening and prolonging its effects, much like selective serotonin reuptake inhibitors (SSRIs) increase serotonin activity to treat depression.
Taken together, the three components of the ECS, the endocannabinoids, their regulatory enzymes and receptors, can be thought of as a key mediator of physiological homeostasis, thus ensuring that various bodily systems function within tight parameters with neither a deficiency nor excess of activity. Just as the immune system deals with invasive proteins from bacteria and viruses, Professor Raphael Mechoulam has hypothesized that the ECS serves an analogous role in the body to neutralize and rectify non-protein insults, such as trauma or oxygen lack.
What if the ECS itself is out of balance? How might this be manifested? Recent discoveries have provided some insights. Ideally, if the ECS is functioning normally, a person might enjoy a normal mental state, without pain, have good digestive function, etc. In contrast, morbid obesity is accompanied by a metabolic syndrome with increased inflammation, insulin resistance and even diabetes. The ECS has been observed to be hyperactive in such states. Similarly, an excess of CB1 activity can be associated with hepatic (liver) fibrosis. Such problems led to the development of drugs such as rimonabant (aka Acomplia® or SR141716) to combat this excess. This drug is an inverse agonist at CB1. That means that it antagonizes the receptor so avidly that it drives down the baseline activity of the ECS, thus lowering what is termed “endocannabinoid tone.” While this might be effective to reduce hunger and weight gain, and improve laboratory findings of the metabolic syndrome, the widespread effects of this drug also spilled over to other systems to produce undesirable adverse events (side effects) such as depression and suicidality that led to its removal from the market. Other liabilities of CB1-inverse agonists would include nausea, an increased likelihood of seizures and even development of malignant tumors. In contrast, CBD is a milder neutral antagonist at CB1 that may be capable of addressing similar medical needs without the attendant risks.
What if endocannabinoid levels are too low? It has been theorized and subsequently borne out in subsequent research that numerous mysterious disorders fit the description of “clinical endocannabinoid deficiency” (CED). Noteworthy among these are migraine, fibromyalgia and idiopathic bowel syndrome (IBS or “spastic colon”). These disorders affect millions of otherwise healthy people who are plagued by chronic pain and other symptoms, leading to extensive medical tests and attempts at treatment, often to limited benefit. The three conditions tend to affect the same individuals at various times of their lives, and are therefore termed “co-morbid.” All three are characterized by “central sensitization,” the concept that normal sensations in the brain are magnified to the point of becoming painful when they would not be to a person free from the affliction. The three disorders also benefit from treatment with cannabinoids according to patient testimonials. Available data confirm that the target organs (brain, gut, musculoskeletal system) seem to express lower than normal levels of anandamide, thus providing credence for the concept that they would benefit from treatments that would upregulate the ECS back to normal levels. Similar putative (theoretical) deficiencies have been highlighted in the ECS for numerous other conditions including intractable depression, posttraumatic stress disorder (PTSD), neuropathic pain conditions such as complex regional pain syndrome, causalgia, post-herpetic neuralgia, interstitial cystitis, and even certain forms of infertility and early miscarriage.
Finally, many forms of cancer are accompanied by increases of CB1 and/or CB2 expression, felt to be part of the body’s effort to combat the disorder. Interestingly, the phytocannabinoids demonstrate the potential to treat cancer in high doses without harming the normal cells of the body. Some of the mechanisms are mediated through CB1 and/or CB2, but others seem to work through independent, non-receptor means. Cancer arises due to a loss of ability for malignant cells to undergo apoptosis, a normal process of programmed cell death whereby the body remolds and renews itself. Instead, cancer cells become immortalized, divide and grow in an uncontrolled fashion, invade surrounding tissues, stimulate their own blood supply, and even metastasize (spread remotely to distant sites). The endo- and phytocannabinoids, particularly CBD, have the ability to reverse or prevent many of these effects, as demonstrated in experiments in many cancer cell types and even in a growing number of case reports in humans. Beyond the issue of eliminating the malignancy itself, properly constituted cannabinoid treatment may hold the promise of additional “side benefits” by simultaneously addressing attendant symptoms of cancer: pain, nausea, sleep disturbance, depression and anxiety.
Throughout human history, most medicines have been derived from plants. This pattern began to change in the 19th century and accelerated in the 20th with the advent of synthetic chemistry. Modern models of drug discovery attempt to identify the key receptor or abnormal gene at the root of a disease process, and then computer-design a potent chemical that will bind to the target region with a high affinity. Sometimes this approach is fruitful, but often attendant toxicities are not identified until far into the development program, and even more often, despite this precise targeting, the drug may not exert sufficient benefits on a complex and chronic disease process to actually improve the patient’s condition. Certain disorders, such as cancer, diabetes and diseases of aging such as Alzheimer disease and osteoporosis require drug combination regimens that affect multiple targets in order to attempt to alleviate their myriad complex problems. What seems necessary are better and safer treatments that address the larger problems of disease pathophysiology and degeneration. Botanicals (plant-based medications) frequently fit this profile quite well in contrast to the “silver bullet” single chemical model that is most prevalent in contemporary Western medicine.
It is historically true that pharmacognosy, the study of medicinal plants, has frequently pointed us in the proper direction to better understand our own body chemistry. Examples are numerous: aspirin, a semi-synthetic derivative of salicylic acid from willow bark was available for 100 years before the basis of its ability to treat pain and inflammation led to the discovery of prostaglandins. Similarly, opium was used for thousands of years before research succeeded in identifying endogenous (within) opioids, the endorphins and enkephalins. Cannabis research similarly resulted in a trail that eventually led to the discovery of the endocannabinoid system, perhaps decades earlier than it might have otherwise been discovered. The future of therapeutics appears much brighter as a result, since an understanding of the ECS portends to offer many more effective and safer remedies for disorders that have previously proven intractable to conventional treatment.
In summary, the endocannabinoid system is a recently discovered regulatory physiological system that holds great promise for improvements in human quality of life. To date, it has not received the attention that it deserves in physician and patient education, nor in research expenditures. Should these shortcomings be rectified, it stands to reason that the public health would benefit enormously.






 Imbalanced Endocannabinoid System
Imbalanced Endocannabinoid System